- Recommended name
- Goniodomin B
- Synonyms
- Goniodomin-B
- Recommended acronym
- GDB
- Abbreviation
Progenitors
No progenitors registered
Vector Species
No vector species registered
- Structure
-
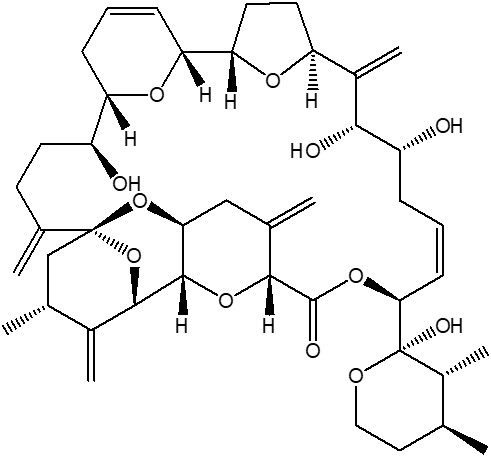
- Formula
- C43H60O12
- Exact mono-isotopic mass
- 768.40848
- Molfile
- see other chem files
- Alternative molfiles
- n/a
- SMILES
- C=C([C@]2([H])CC[C@@](O2)([H])[C@@](O3)([H])C=CC[C@]37[H])[C@@H](O)[C@H](O)C\C=C/[C@H](OC([C@@](O4)([H])C(C[C@H]5[C@@]4([H])[C@@H](O6)C([C@H](C)C[C@@]6(C(CC[C@@H]7O)=C)O5)=C)=C)=O)[C@@]1(O)[C@H](C)[C@@H](C)CCO1
- Alternative SMILES
- n/a
- InChi key
- SUFFTSRDKPVOOK-CONSMQGSSA-N
- Alternative InChi keys
- n/a
- InChi
- InChI=1S/C43H60O12/c1-22-18-19-49-43(48,28(22)7)36-13-8-10-30(45)37(46)27(6)31-16-17-34(50-31)33-12-9-11-32(51-33)29(44)15-14-25(4)42-21-24(3)26(5)39(55-42)40-35(54-42)20-23(2)38(53-40)41(47)52-36/h8-9,12-13,22,24,28-40,44-46,48H,2,4-6,10-11,14-21H2,1,3,7H3/b13-8-/t22-,24+,28+,29-,30+,31-,32-,33+,34-,35-,36-,37+,38+,39-,40-,42+,43+/m0/s1
- Alternative InChis
- Spectra available
- True
- Chem files
- chemfiles/Goniodomin_B_Takeda.mol chemfiles/Goniodomin_B_Takeda.cdx
References
- Harris et al., 2020
- Harris, C. M., K. S. Reece and T. M. Harris (2020). "Revisiting the toxin profile of Alexandrium pseudogonyaulax; Formation of a desmethyl congener of goniodomin A." Toxicon 188: 122-126.
- Harris et al., 2021
- Harris, C. M., B. Krock, U. Tillmann, C. J. Tainter, D. F. Stec, A. J. C. Andersen, T. O. Larsen, K. S. Reece and T. M. Harris (2021). "Alkali Metal- and Acid-Catalyzed Interconversion of Goniodomin A with Congeners B and C." Journal of Natural Products 84(9): 2554-2567.
- Certified
- False
- Certified links
-
-
n/a
- Non certified reference material
- False
Chemical analysis
- Research
- True
- Standardized
- Unknown
- Validated
- Unknown
- Official
- n/a
Structure recognition assays
- Research
- Unknown
- Standardized
- Unknown
- Validated
- Unknown
- Official
- n/a
Functional assays
- Research
- Unknown
- Standardized
- Unknown
- Validated
- Unknown
- Official
- n/a
Animal assays
- Research
- Unknown
- Standardized
- Unknown
- Validated
- Unknown
- Official
- n/a
References
- Krock et al., 2018
- The compound initially described by Krock et al., 2018, as GDB was later found to be actually 34-desmethyl-GDA. GDA and GDB are isobaric. See: Harris, C. M., K. S. Reece and T. M. Harris (2020). "Revisiting the toxin profile of Alexandrium pseudogonyaulax; Formation of a desmethyl congener of goniodomin A." Toxicon 188: 122-126.
- Harris et al., 2023
- Transformation of GDA into GDB, GDC and corresponding seco acids highly depends on pH of the mobile stationary phase and the adduct ion (ammonium vs. sodium). Most unaltered GD profiles are obtained by alkaline mobile phases and ammonium adducts.
- Regulatory status
- False
- Human toxic syndrome(s)
- n/a
- Organ system toxicity
- n/a
- Risk assessment
- Unknown
- Molecular targets known
- True
- Molecular targets
- F-actin, G-actin
- Toxic to aquatic animals
- Unknown
- TEF available
- False
- Notes
References
- Harris et al., 2021
- GDB is most likely not prodcuded by dinoflagellates, but an rapid transformation product of GDA by conatct with acids and alkali metals. See: Harris, C. M., B. Krock, U. Tillmann, C. J. Tainter, D. F. Stec, A. J. C. Andersen, T. O. Larsen, K. S. Reece and T. M. Harris (2021). "Alkali Metal- and Acid-Catalyzed Interconversion of Goniodomin A with Congeners B and C." Journal of Natural Products 84(9): 2554-2567.